electron geometry chart|electron pair geometry vs molecular geometry : Tuguegarao Learn how to use VSEPR Theory to organize molecules based on their geometric structures. See examples of linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral, bent, trigonal pyramidal, seesaw, T .
2022 NBA championship odds: Lakers, Nets co-favorites to win it all next season; Bucks have fourth-best odds The Suns come in with the fifth-best odds
PH0 · molecular geometry chart with angles
PH1 · electron pair geometry vs molecular geometry
PH2 · electron geometry table
PH3 · electron geometry calculator
PH4 · electron geometry and hybridization chart
PH5 · electron domain geometry table
PH6 · electron domain geometry chart
PH7 · electron and molecular geometry table
PH8 · Iba pa
MOREEE BOLD!💦 @pinayleakedvids
electron geometry chart*******This article explains the molecular geometry, which is the three-dimensional structure or arrangement of atoms in a molecule. It covers how to determine the shapes of molecules using Lewis electron dot structures and valence-shell electron-pair repulsion (VSEPR) theory, as well as bond angles . Tingnan ang higit pa
The three-dimensional structure or arrangement of atoms in a molecule is called molecular geometry. Understanding the . Tingnan ang higit paThe valence shell electron pair repulsion theory states that electrons will spread themselves as far from each other as possible to minimize repulsion between them whether they are in bond pairs or lone pairs on center atom. It predicts distribution . Tingnan ang higit pa
To determine the shapes of molecules we must become familiar with the Lewis electron dot structure which helps us identify bond pairs and lone pairs. Then apply valence-shell electron-pair repulsion (VSPER) theory to determine molecular geometry . Tingnan ang higit paA polar molecule has two poles because its electrons are not distributed equally resulting in difference electronegativity among . Tingnan ang higit paLearn how to use VSEPR Theory to organize molecules based on their geometric structures. See examples of linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral, bent, trigonal pyramidal, seesaw, T .Determine the Electron geometry from the Lewis dot structure. Determine the molecular geometry. It is very important from the onset that students understand the difference between electronic geometry .
electron pair geometry vs molecular geometryWe can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom, ignoring .A table that shows the number of electron groups, lone pairs, and electron pair arrangement for different molecular geometries. It also includes the approximate bond angles for .
The Electron Domain Geometries. Molecular Geometries from each Electron Domain Geometry . Since electron pairs cannot be seen, the electron domain geometries are .VSEPR Theory. Table Of Contents. Postulates. Basic Molecular Structures. AXE Notation. Predicting the Molecular Geometry. Limitations. Lewis structure is a straightforward way of representing .
Learn how to identify the molecular geometry and bond angles of a molecule using the VSEPR model. See examples of linear, trigonal, tetrahedral, and bent geometries and their corresponding bond angles.
Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or .The electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. The molecular geometry is the shape of the molecule. So when asked to describe the .
Electron Geometry: Describes the arrangement of bonds and lone pairs around a central atom. . to the central atom, boron. Based on the chart, the molecular geometry for BF 3 would be trigonal planar, with an .
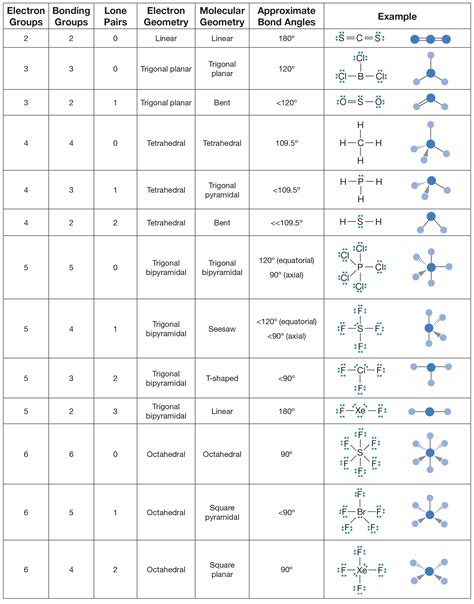
Electron-pair Geometry versus Molecular Structure. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in Figure 7.16 describe all regions where electrons are located, bonds as well as lone pairs. Molecular structure describes the location of the .
We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence .
We recommend using the latest version of Chrome, Firefox, Safari, or Edge. Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!Molecular geometry is the name of the geometry used to describe the shape of a molecule. The electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. The molecular geometry is the shape of the molecule. So when asked to describe the shape of a molecule we must respond with a .

The molecular geometry, or three-dimensional shape of a molecule or polyatomic ion, can be determined using valence-shell electron-pair repulsion (abbreviated VSEPR and pronounced “VES-per”) theory, in which the basic principle is valence electrons around a central atom stay as far apart as possible to. minimize the repulsions.
electron geometry chart electron pair geometry vs molecular geometry The electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{CH_4}\). In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. Although the lone pair is not visible, it will affects the location and bond angles among other atoms in the molecule.Electronic Geometry, Molecular Shape, and Hybridization Page 1 The Valence Shell Electron Pair Repulsion Model (VSEPR Model) The guiding principle: Bonded atoms and unshared pairs of electrons about a central atom are as far from one another as possible. Bonded atoms Nonbonded Pairs Total Electronic Geometry Molecular
The best UK online casino sites often accept numerous payment methods that their members can use. In addition to card payments with the likes of Visa and Mastercard, these casinos will let you fund your account with Skrill, Neteller, PayPal and .
electron geometry chart|electron pair geometry vs molecular geometry